Product Support
For thirty years, we have been dedicated to developing, building and supporting high quality and robust tools to help diagnose lead poisoning. Supporting our customers, and the patients who depend on them is at the heart of our business.
For thirty years, we have been dedicated to developing, building and supporting high quality and robust tools to help diagnose lead poisoning. Supporting our customers, and the patients who depend on them is at the heart of our business.
Register Your LeadCare II
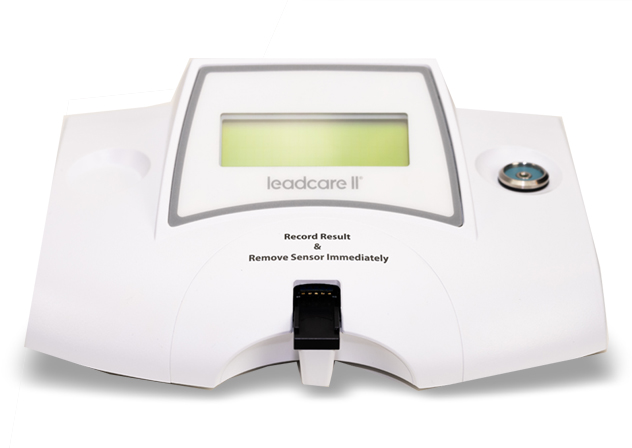
Getting Started with Your LeadCare® II
LeadCare® II is the only CLIA-waived, point-of-care system for blood lead testing.
To use the LeadCare II System, a facility must have:
- Have a CLIA Certificate of Waiver or higher;
- Follow the manufacturer’s instructions for test performance and quality control
- Comply with applicable state laws, which may have additional registration, reporting or proficiency requirements.
For more information to help you determine your state specific requirements to begin blood lead testing with the LeadCare II System, contact the Product Support Team at (800) 275-0102 or email us at LeadCareSupport@meridianbioscience.com.
Additionally, visit the Center for Disease Control and Prevention’s (CDC) Healthy Homes Map to identify the department responsible for lead testing in your state.
How to Obtain a CLIA Certificate
Offices or laboratories operating under a CLIA Certificate of Waiver (or higher) can perform the LeadCare® II test in accordance with the manufacturer’s instructions. This page includes information to assist you in the pursuit of a CLIA Certificate.
- Learn the basics about a CLIA Certificate of Waiver and how to obtain one: CMS publication: How to Obtain a Certificate of Waiver
- Download a CLIA Application for Certification
Submit your completed form to:
Alabama Department of Public Health
Division of Health Care Facilities
CLIA Program
P.O. Box 303017
Montgomery, AL 36130-3017
Phone: (334) 206-5120
Fax: (334) 206-5303
Some address