Information on the LeadCare Test Kit “Controls Out of Range-Low”
(“COOR-LO”) Recall
We are pleased to announce that Magellan Diagnostics has resumed distribution of LeadCare® II, LeadCare Plus®, and LeadCare Ultra®.
LeadCare II resumed distribution beginning the week of February 14th, 2022. LeadCare Plus and Ultra resumed distribution as of October 14th, 2022.
Please note that the shelf life of LeadCare II, Plus, and Ultra product is currently 9 months from the date of manufacture. The electronic calibration button in each kit will not permit that lot number to be used past the expiration date listed on the kit box label.
“COOR-LO” Recall Overview
The Magellan quality management system identified discrepancies in the performance of LeadCare test kits. It was originally suspected that a defect with the plastic used to manufacture Treatment Reagent tubes was the source of this discrepancy; however, studies have shown that the paperboard material used to package Treatment Reagent tubes is the source of the contaminant. As the issue had the potential to underestimate blood lead levels when processing patient samples, Magellan voluntarily recalled test kits and temporarily suspended shipping. Magellan has conducted numerous studies and experiments to understand the root cause of the issue and has identified suitable replacement materials such that shipments can resume.
Please note LeadCare Analyzers (LeadCare® II, LeadCare Plus®, and LeadCare Ultra®) ARE NOT impacted by this recall. You are still able to use this instrumentation.
Please review the test kit LOT numbers below that are impacted by the recall.
Product Name |
LeadCare™ II Blood Lead Test Kit |
LeadCare™ Plus Blood Lead Test Kit |
LeadCare™ Ultra Blood Lead Test Kit |
|
Catalog Number |
70-6762 | 82-0004 | 70-8098 | |
UDI |
N/A | N/A | N/A | |
RecalledLot Numbers |
Initial |
2013M, 2014M 2015M, 2016M, and 2017M | 2011MU | |
Expanded (2nd) |
2101M, 2103M, 2105M, 2106M and 2107M | 2104MU and 2108MU | ||
Expanded (3rd) |
2012M Sublots: -08, -09, -10, -11, -12, -13, and -14 | N/A | ||
| Full Lots: 2018M, 2102M, 2109M, 2110M, 2111M, 2112M, 2113M, 2114M, 2115M, and 7114M | ||||
Magellan Reference No. |
1218996-05/07/2021-0001R | |||
| FDA Recall Notifications: | Lots Impacted in the Initial COOR-LO Recall:
|
CDC Information Related to the COOR-LO Recall:
NOTE: This CDC alert, dated October 14, 2021, does not add any new lots to the recall but rather reports on the lots indicated in the table posted above per Magellan’s August 30, 2021 expansion of the recall. |
Lots Impacted in the Expanded (2nd) COOR-LO Recall:
|
| Press Release: Magellan Diagnostics, Inc. Expands Voluntary Recall of LeadCare® Test Kits | Lots Impacted in the Expanded (3rd) COOR-LO Recall:
|
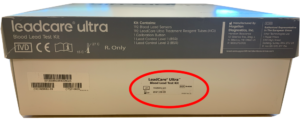
Where to find the lot number on your LeadCare Test Kit box
|
LeadCare® II
|
LeadCare Plus®
|
LeadCare Ultra®
|
Contact Information
If you have any questions, please call Magellan’s LeadCare Product Support Team at 1-800-275-0102, or by email at LeadCareSupport@magellandx.com